|
Strain Name
|
C57BL/6JNifdc DIO
|
Common Name
|
B-DIO mice
|
|
Background
|
C57BL/6JNifdc
|
Catalog number
|
112938
|
|
Aliases
|
/
|
Body Weight
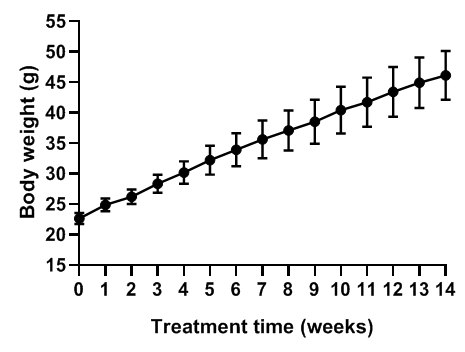
Body weight of B-DIO mice. Male C57BL/6JNifdc mice, aged 5 weeks, were put on a diet consisting of 60 kcal% high-fat feed. Their weights were recorded weekly from a total of 116 mice. The data is presented as Mean ± SD. After being fed with the high-fat diet for 10 weeks, the average weight of the B-DIO mice exceeded 40g.
Glucose tolerance tests
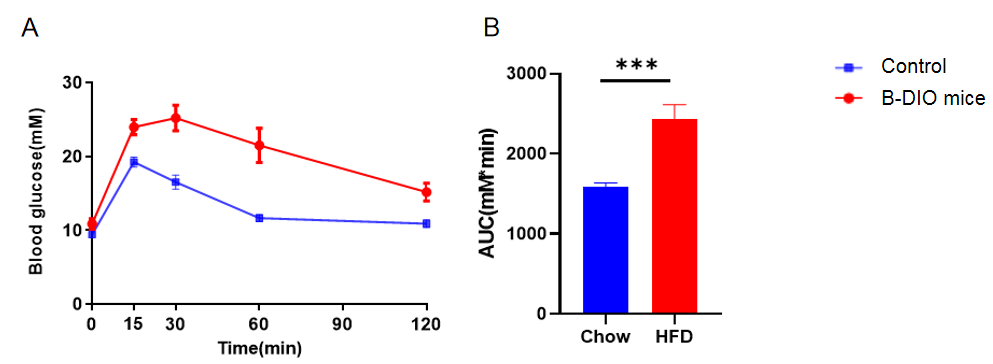
Glucose tolerance tests of B-DIO mice. Male B-DIO mice were fed a high-fat diet for 12 weeks. After fasting for 16 hours, 10 mice were intraperitoneally injected with 2 g/kg D-Glucose. Blood glucose levels were measured at the following time points after injection: 15 minutes, 30 minutes, 60 minutes, and 120 minutes. (A) Glucose tolerance ability after HFD induction. (B) Area under curve. Data was shown as Mean ± SEM. (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001)
Blood biochemical analysis
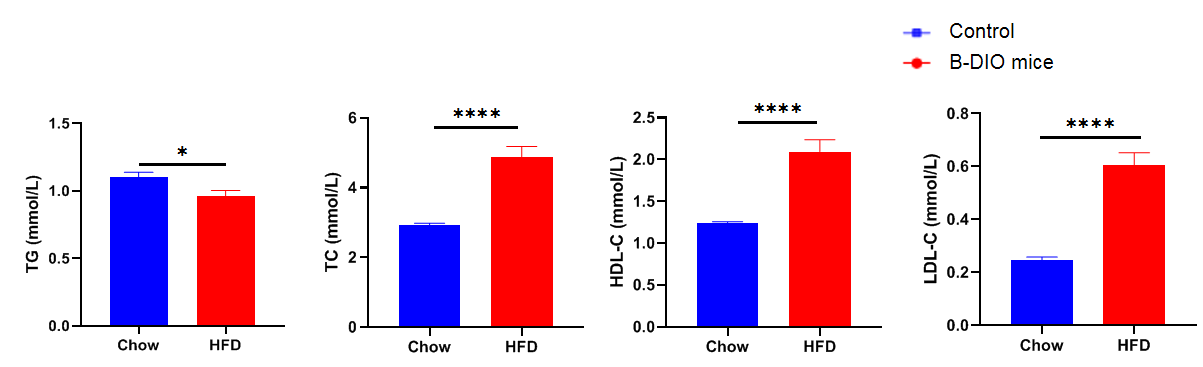
Blood biochemical analysis in B-DIO mice. Male B-DIO mice were fed a high-fat diet for 12 weeks. Plasma concentrations of TG, TC, LDL-C, and HDL-C in B-DIO mice (HFD) and wild-type C57BL/6 mice with chow (control) were analyzed. The TC, HDL-C, LDL-C of B-DIO mice is significantly higher than that of control. Data was shown as Mean ± SEM, n=10 mice per group. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
TG, triglycerides; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
In vivo efficacy of Semaglutide in B-DIO mice
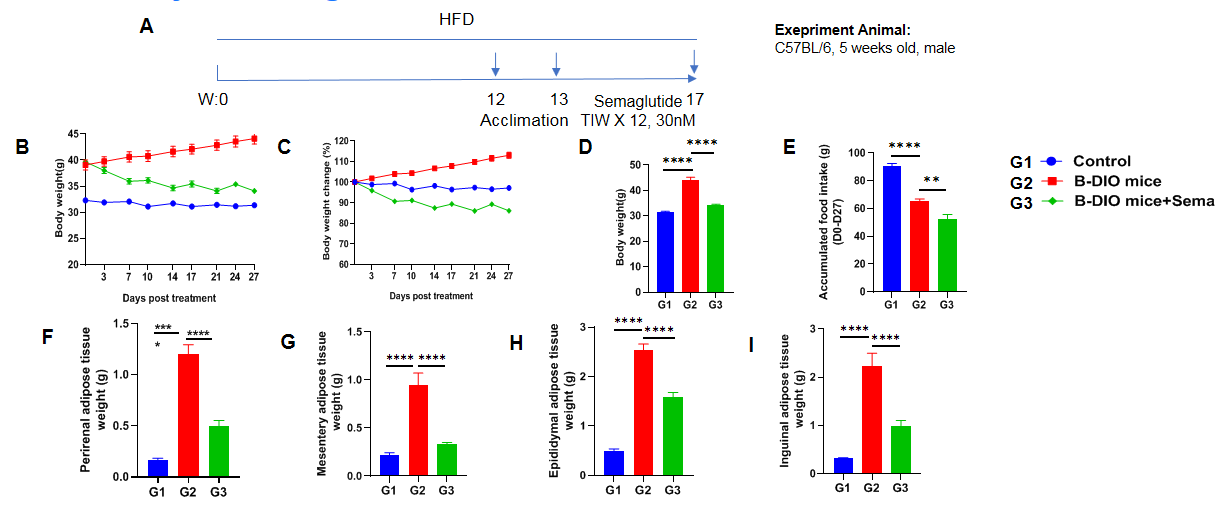
Anti-obesity effect of Semaglutide in B-DIO mice. (A) Male B-DIO mice were fed a high-fat diet for 12 weeks to induce obesity. After a week of acclimation feeding due to transportation, Semaglutide was administered via subcutaneous injection thrice per week for 4 weeks after grouping. (B-D) Body weight change and terminal body weight after Semaglutide treatment. (E) Accumulated food intake during treatment. (F-I) Adipose tissue weights after treatment. Data are expressed as mean ± SEM. N=10 mice per group. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
In vivo efficacy of Semaglutide in B-DIO mice
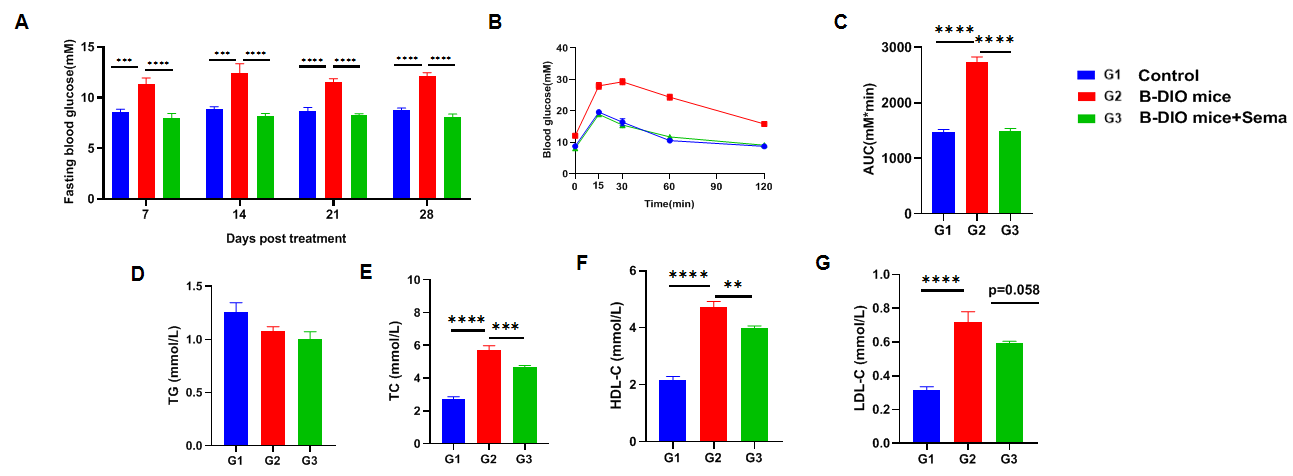
The hypoglycemic and lipid-lowering effects of Semaglutide in B-DIO mice. (A) Blood glucose change after Semaglutide treatment. (B) Glucose tolerance ability after treatment. (C) Area under curve of B. (D-G) Blood biochemical analysis after treatment. Data are expressed as mean ± SEM. N=10 mice per group. *p<0.05, **p<0.01, ***p<0.001 ,****p<0.0001.
In vivo efficacy of Dulaglutide in B-DIO mice
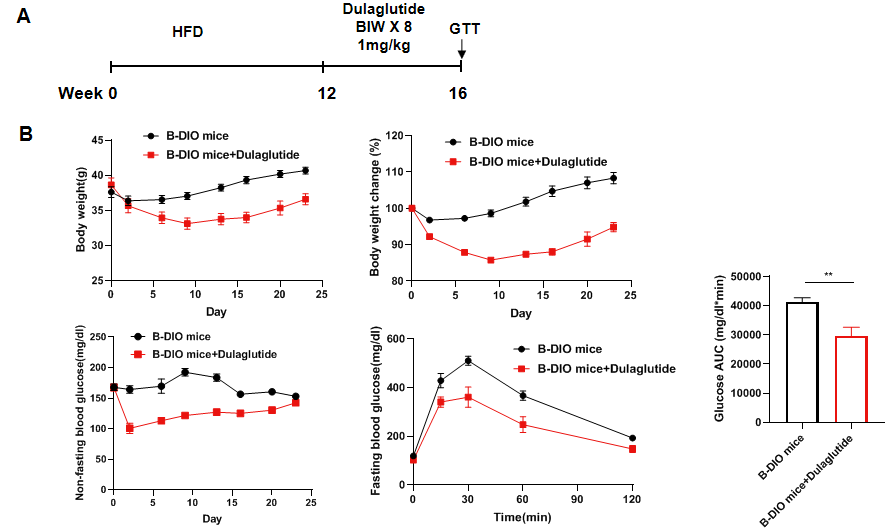
Anti-obesity effect of Dulaglutide in B-DIO mice. (A) Male B-DIO mice were fed a high-fat diet for 12 weeks to induce obesity. Dulaglutide (in house) was administered via subcutaneous injection twice per week for 4 weeks after grouping. (B) Glucose tolerance test were performed the day after the last dose (n=8 mice per group). After treatment with Dulaglutide, both non-fasting and fasting blood glucose levels were reduced compared to those in B-DIO mice. Values are expressed as mean ± SEM.

















