| Strain Name |
C57BL/6-Pdcd1tm1(PDCD1)BcgenCd274tm1(CD274)BcgenTnfrsf1btm1(TNFRSF1B)Bcgen/Bcgen
|
Common Name | B-hPD-1/hPD-L1/hTNFR2 mice |
| Background | C57BL/6 | Catalog number |
130849 |
|
Related Genes |
PD-1 (Programmed death-1) CD274 (CD274 antigen) TNFRSF1B (Tumor necrosis factor receptor superfamily, member 1b) |
||
|
NCBI Gene ID |
18566,60533,21938 | ||
Phenotypic analysis
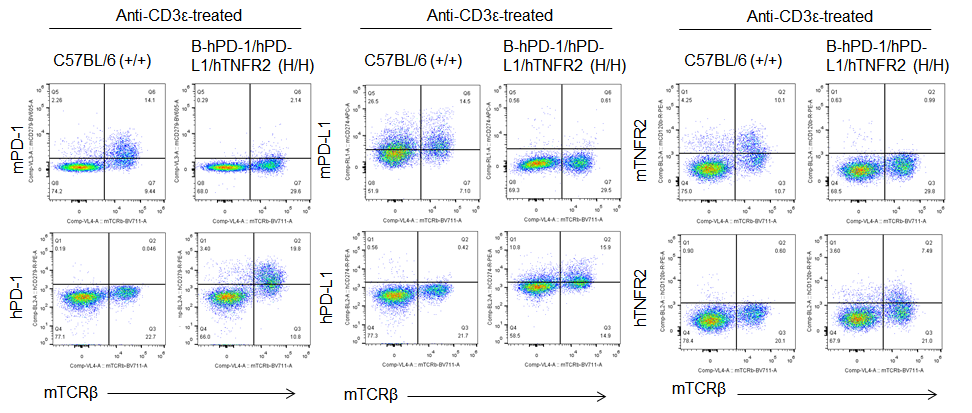
Protein expression analysis in T cells
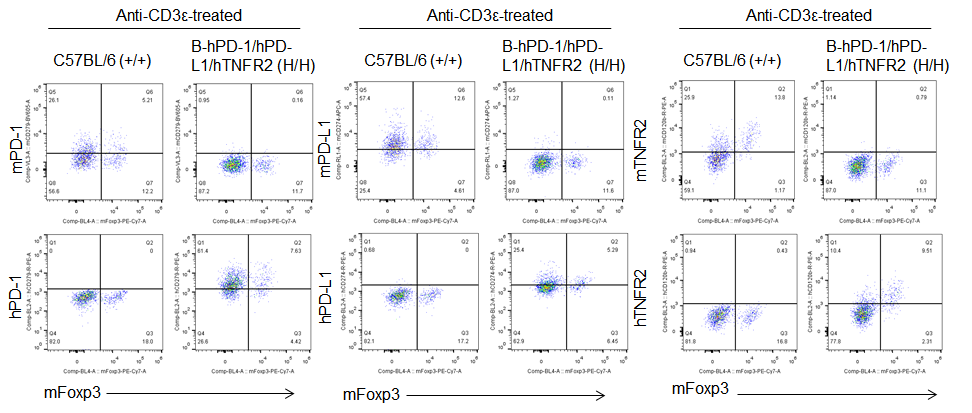
Protein expression analysis in Treg cells
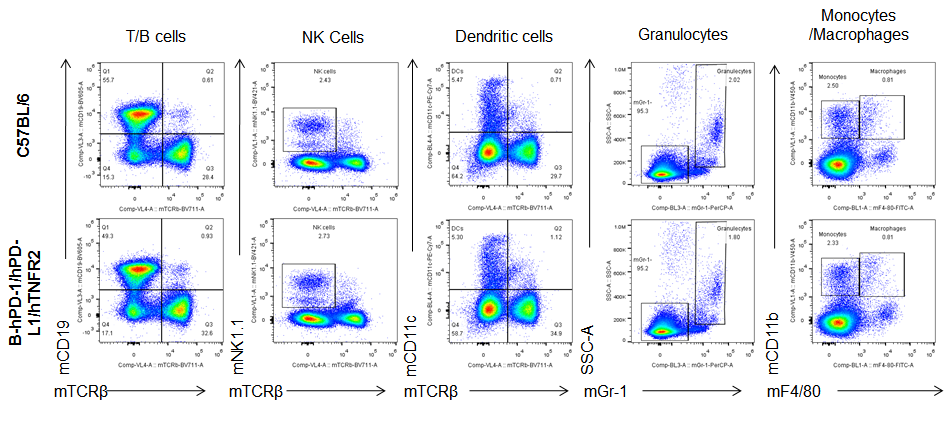
Analysis of blood leukocytes cell subpopulations in B-hPD-1/hPD-L1/hTNFR2 mice
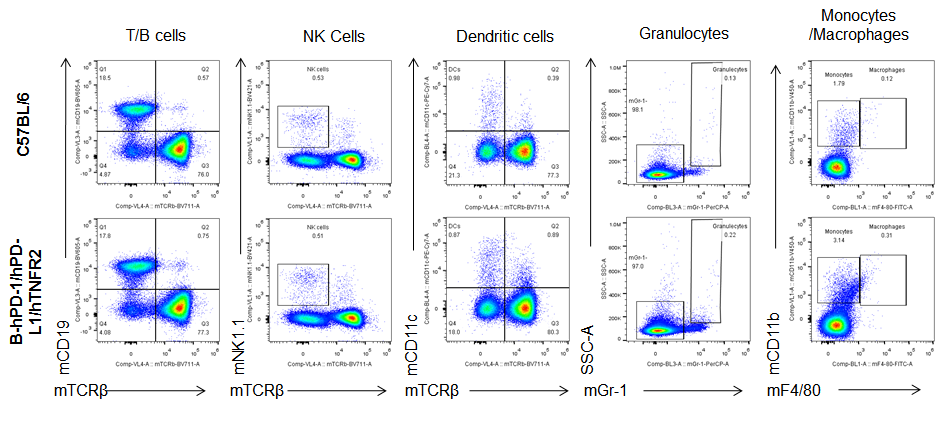
Analysis of spleen leukocytes cell subpopulations in B-hPD-1/hPD-L1/hTNFR2 mice
Analysis of lymph node leukocytes cell subpopulations in B-hPD-1/hPD-L1/hTNFR2 mice
Analysis of blood, spleen and lymph node leukocytes cell subpopulations in B-hPD-1/hPD-L1/hTNFR2 mice
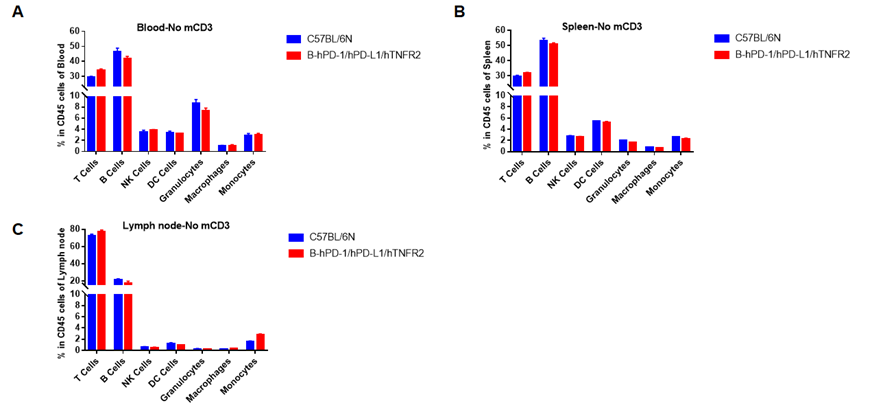
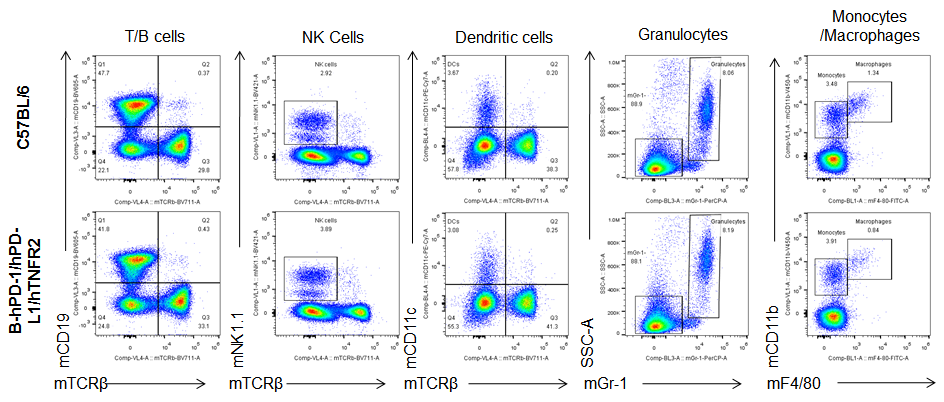
Analysis of blood, spleen and lymph node leukocytes cell subpopulations by FACS. Blood, spleen and lymph node leukocytes cell were isolated from female mice in the panel(n=3, 6 week-old). Flow cytometry analysis was performed to assess leukocyte subpopulations. Percent of T, B, NK, Granulocytes, Monocyte, DC and macrophage cells in homozygous B-hPD-1/hPD-L1/hTNFR2 mice were similar to those in the C57BL/6 mice, demonstrating that the humanized mouse does not change the overall development, differentiation or distribution of these cell types in blood, spleen and lymph node.
Analysis of blood, spleen, lymph node T cell subpopulations in B-hPD-1/hPD-L1/hTNFR2 mice
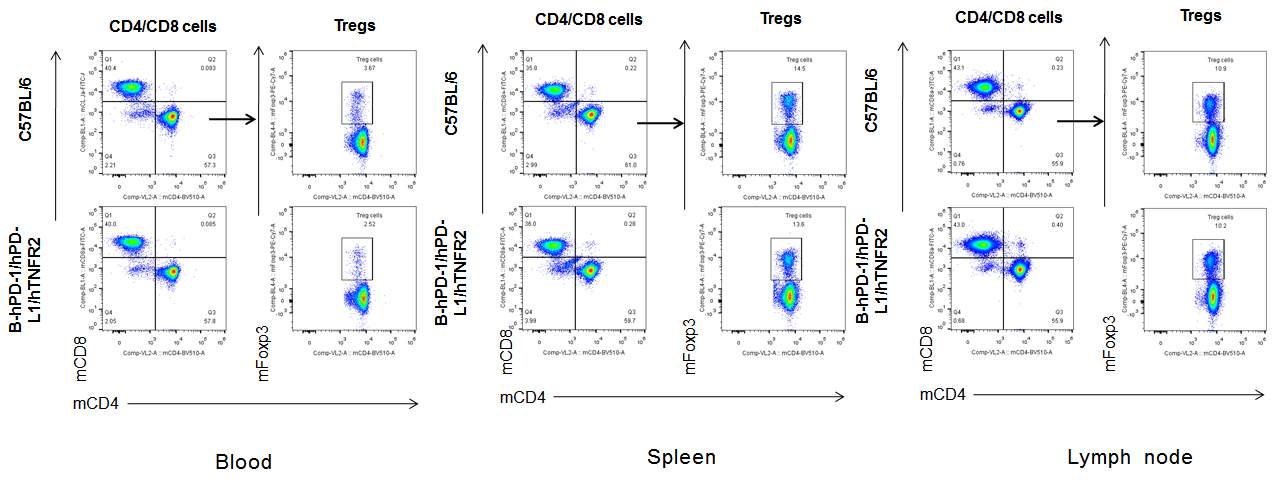
Analysis of blood, spleen and lymph node T cell subpopulations in B-hPD-1/hPD-L1/hTNFR2 mice
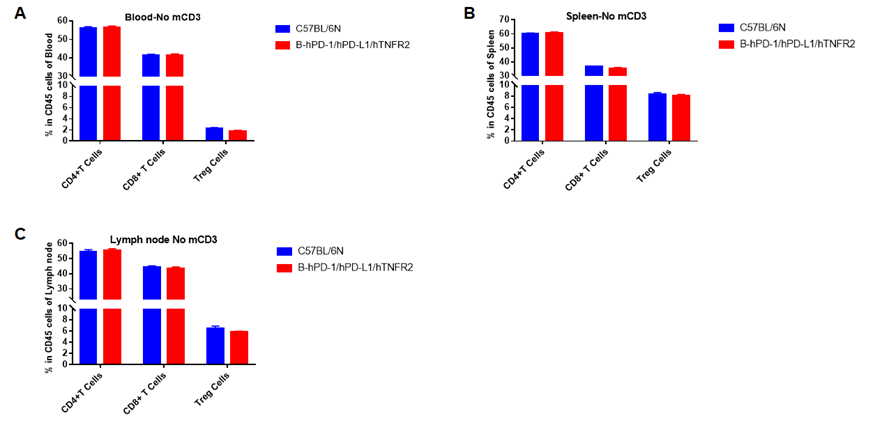
Analysis of blood, spleen and lymph node T cell subpopulations by FACS. Blood, spleen and lymph node leukocytes cell were isolated from female mice in the panel(n=3, 6 week-old). Flow cytometry analysis was performed to assess leukocyte subpopulations. Percent of CD4+T, CD8+T and Tre cells in homozygous B-hPD-1/hPD-L1/hTNFR2 mice were similar to those in the C57BL/6 mice, demonstrating that the humanized mouse does not change the overall development, differentiation or distribution of these cell types in blood, spleen and lymph node.
Blood routine test of B-hPD-1/hPD-L1/hTNFR2 mice
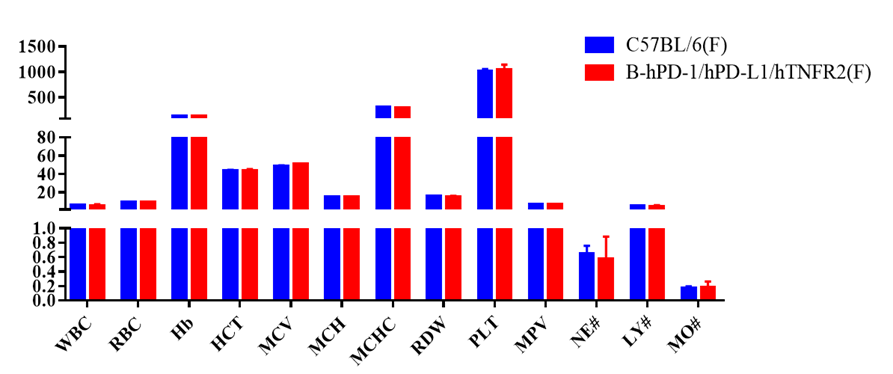
Complete blood count (CBC). Blood from C57BL/6 and B-hPD-1/hPD-L1/hTNFR2 mice (n=5, 6 week-old, female) were collected and analyzed for CBC. Any measurement of B-hPD-1/hPD-L1/hTNFR2 mice in the panel were similar to C57BL/6, indicating that humanized mouse does not change blood cell composition and morphology. Values are expressed as mean ± SEM.
Blood chemistry of B-hPD-1/hPD-L1/hTNFR2 mice
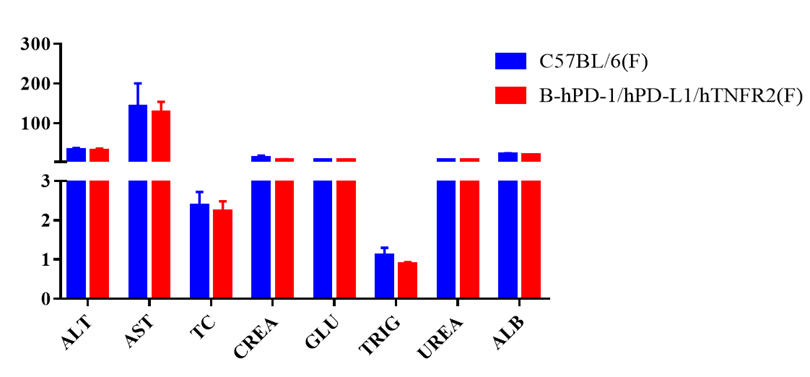
Blood chemistry tests of B-hPD-1/hPD-L1/hTNFR2 mice. Serum from C57BL/6 and B-hPD-1/hPD-L1/hTNFR2 mice (n=5, 6 week-old, female) were collected and analyzed for levels of ALT, AST and other indicators in the panel. There was no differences on either measurement between C57BL/6 and humanized mouse, indicating that humanized mouse does not change ALT and AST levels or health of liver. Values are expressed as mean ± SEM.
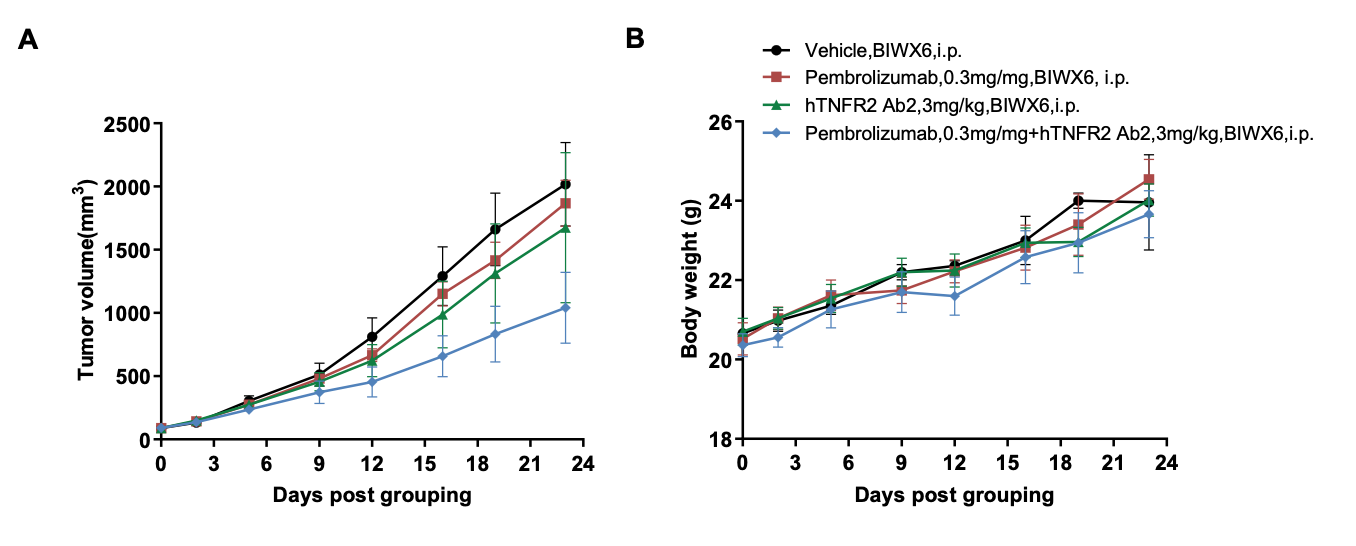
Combination therapy of anti-human PD-1 and anti-human TNFR2 antibody