|
Common name |
B-hCLDN18.2 A549 | Catalog number | 310791 |
| Aliases | OASFTA5, SFTPJ | Disease | Carcinoma |
|
Organism |
Human | Strain | -- |
| Tissue types | Lung | Tissue | Lung |
Description
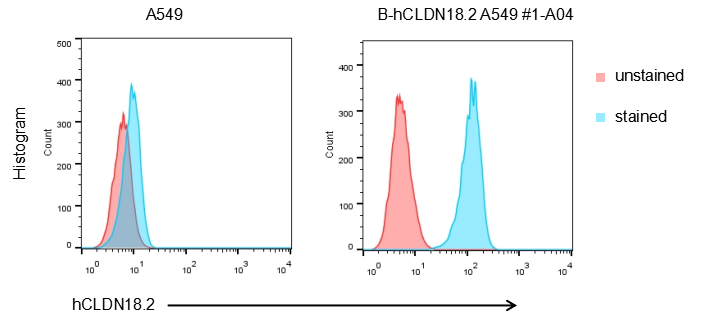
The human CLDN18.2 coding sequence was inserted into the AAVS1 locus in A549 cells. Human CLDN18.2 is highly expressed on the surface of B-hCLDN18.2 A549 cells.
Application
B-hCLDN18.2 A549 cells have the capability to establish tumors in vivo and can be used for efficacy studies.
Targeting strategy
Gene targeting strategy for B-hCLDN18.2 A549 cells. The exogenous promoter and human CLDN18.2 coding sequence was inserted into the AAVS1 locus wild-type A549.
Tumor growth curve & Body weight changes
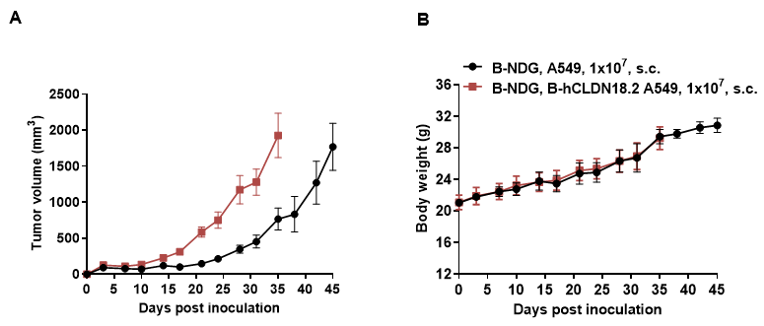
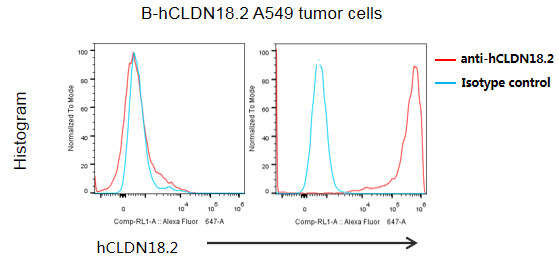
B-hCLDN18.2 A549 cells were subcutaneously transplanted into B-NDG mice (n=5). At the end of the experiment, tumor cells were harvested and assessed for human CLDN18.2 expression by flow cytometry. As shown, human CLDN18.2 was highly expressed on the surface of tumor cells. Therefore, B-hCLDN18.2 A549 cells can be used for in vivo efficacy studies of novel CLDN18.2 therapeutics.
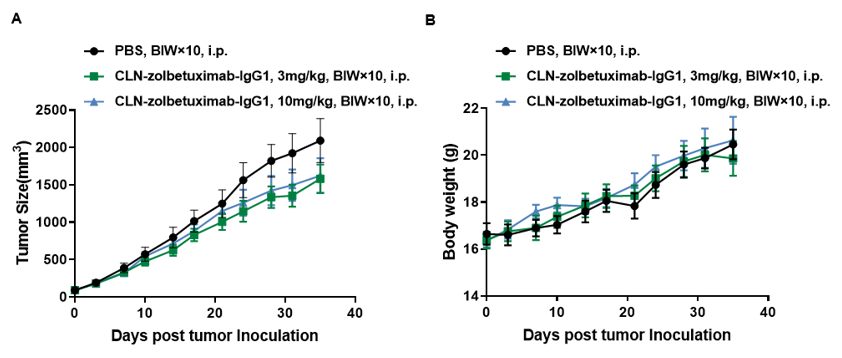
Antitumor activity of anti-hCLDN18.2 antibodies in CB-17 SCID mice. (A) Anti hCLDN18.2 antibody slightly inhibited A549-hCLDN18.2 tumor growth in CB-17 SCID mice. B-CAG-hCLDN18.2 A549 cells were subcutaneously implanted into CB-17 SCID mice (female, 7 week-old, n=5). Mice were grouped when tumor volume reached approximately 90 mm3, at which time they were treated with anti-hCLDN18.2 antibody with different doses and schedules indicated in panel (B) Body weight changes during treatment. As shown in panel A, anti-hCLDN18.2 antibody was efficacious but mild, demonstrating that B-CAG-hCLDN18.2 A549 cells can be used to establish tumor model and provide a powerful preclinical model for in vivo evaluation of anti-hCLDN18.2 antibodies. Values are expressed as mean ± SEM.











