Common name
|
B-hCLDN18.2 MC38
|
Catalog number
|
310715
|
|
Aliases
|
SFTA5, SFTPJ
|
Disease
|
Colon carcinoma
|
Organism
|
Mouse
|
Strain
|
C57BL/6
|
|
Tissue types
|
Colon
|
Tissue
|
Colon
|
Description
The human CLDN18.2 coding sequence was inserted into the ROSA26 locus in MC38 cells. Human CLDN18.2 is highly expressed on the surface of B-hCLDN18.2 MC38 cells.
Application
B-hCLDN18.2 MC38 cells have the capability to establish tumors in vivo and can be used for efficacy studies.
Targeting strategy
Gene targeting strategy for B-hCLDN18.2 MC38 cells. The exogenous promoter and human CLDN18.2 coding sequence was inserted into the ROSA26 locus in murine
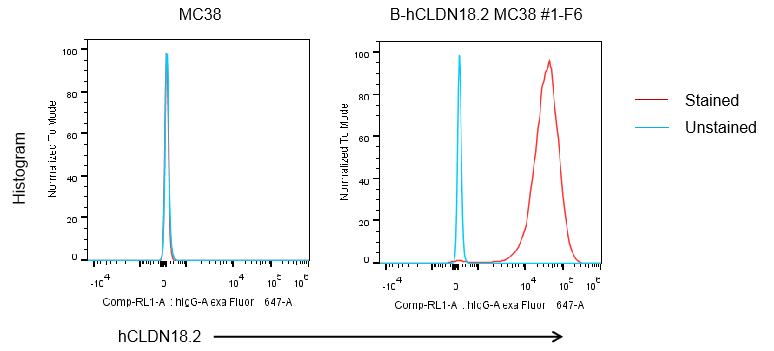
Protein expression analysis
CLDN18.2 expression analysis in B-hCLDN18.2 MC38 cells by flow cytometry. Single cell suspensions from wild-type MC38 and B-hCLDN18.2 MC38 cultures were stained with species-specific anti-CLDN18.2 antibody. Human CLDN18.2 was detected on the surface of B-hCLDN18.2 MC38 cells but not wild-type MC38 cells. The 1-F6 clone of B-hCLDN18.2 MC38 cells was used for in vivo experiments.
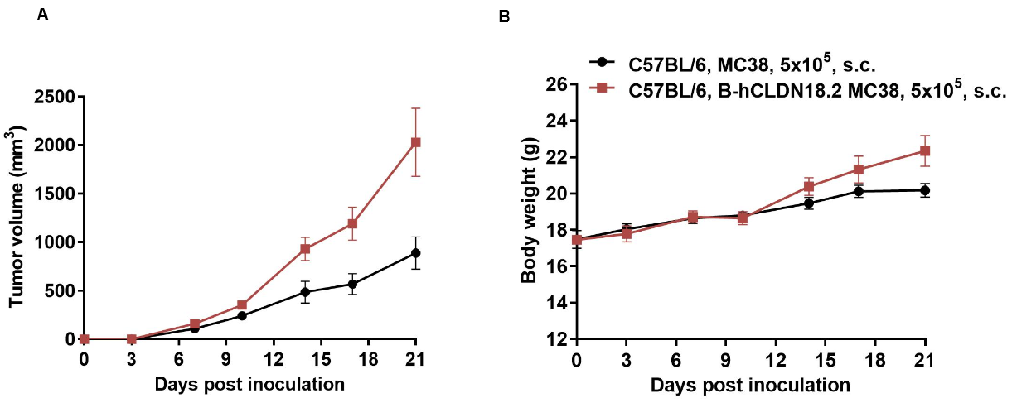
Tumor growth curve & Body weight changes
Subcutaneous homograft tumor growth of B-hCLDN18.2 MC38 cells. B-hCLDN18.2 MC38 cells (5x105) and wild-type MC38 cells (5x105) were subcutaneously implanted into C57BL/6 mice (female, n=5). Tumor volume and body weight were measured twice a week. (A) Average tumor volume ± SEM. (B) Body weight (Mean± SEM). Volume was expressed in mm3 using the formula: V=0.5 X long diameter X short diameter2. As shown in panel A, B-hCLDN18.2 MC38 cells were able to establish tumors in vivo and can be used for efficacy studies.
Protein expression analysis in tumor cells
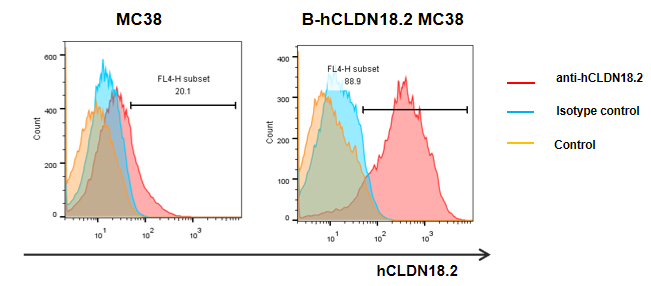
The CLDN18.2 expression analysis in tumor by flow cytometry. B-hCLDN18.2 MC38 cells was transplanted into C57BL/6 mice (n=5), and on 21 days post inoculation, certain volumes of tumors was taken to detect the human CLDN18.2 expression. As shown in the panel, human CLDN18.2 protein was expression on the surface of B-hCLDN18.2 MC38 tumor cells.
Tumor Growth Curve & Body Weight Changes
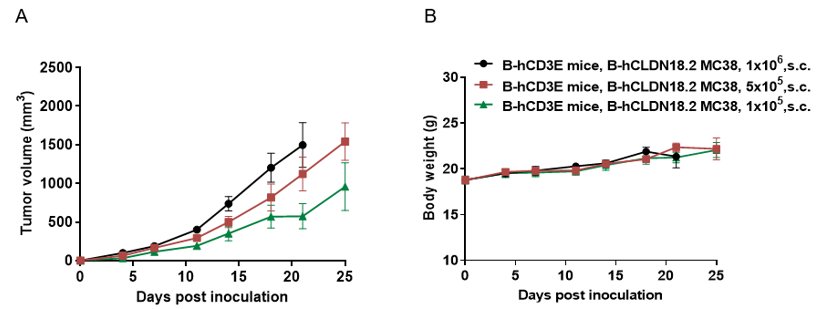
Subcutaneous xenograft tumor growth of B-hCLDN18.2 MC38 cells. B-hCLDN18.2 MC38 cells were subcutaneously implanted into the B-hCD3E mice (female, n=6).Tumor size and mice body weight were measured twice a week. (A) Tumor average volume ± SEM, (B) Body weight (Mean± SEM). Volume was expressed in mm3 using the formula: V=0.5a X b2, where a and b were the long and short diameters of the tumor, respectively. As shown in panel A, B-hCLDN18.2 MC38 were able to establish tumor in vivo and can be used for efficacy study.
Tumor growth curve of individual mice
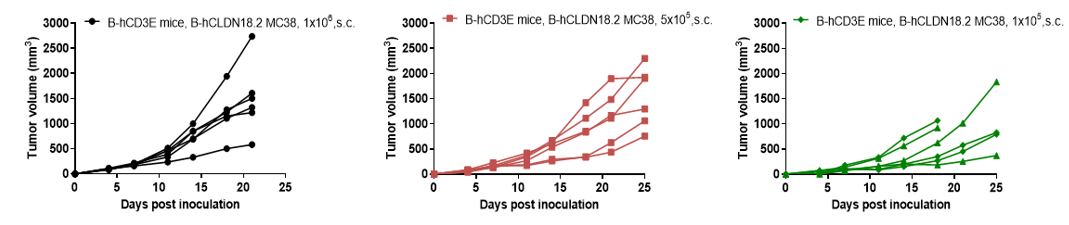
B-hCLDN18.2 MC38 tumor growth of individual mice. B-hCLDN18.2 MC38 cells were subcutaneously implanted into the B-hCD3E mice (female, n=6).Tumor size and mice body weight were measured twice a week. As shown in panel, B-hCLDN18.2 MC38 cells were able to establish tumors in vivo and can be used for efficacy studies.
IHC staining
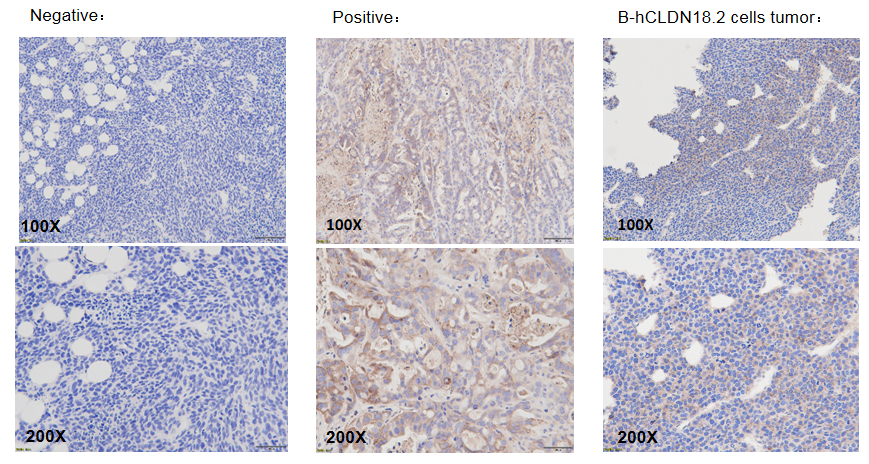
The CLDN18.2 expression analysis in tumor by IHC. B-hCLDN18.2 MC38 cells was transplanted into B-hCD3E mice (n=5), and on days 18 post inoculation, certain volumes of tumors as was taken to detect the human CLDN18.2 expression by IHC. The background of the negative control group was clear. Tumor tissue which expresses human CLDN18.2 protein as a positive control. As shown in the panel, the human CLDN18.2 protein was detection in B-hCLDN18.2 MC38 tumor cells.
In vivo efficacy of anti human CLDN18.2 antibodies
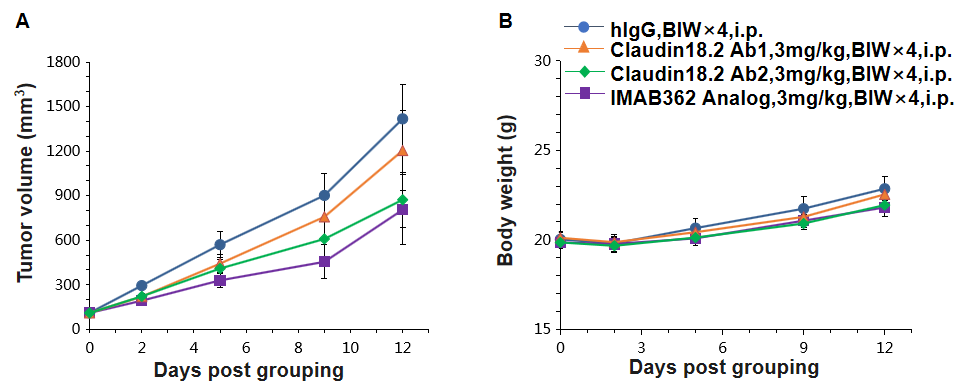
Antitumor activity of anti-human CLDN18.2 antibodies in C57BL/6 mice. (A) Anti human CLDN18.2 antibodies inhibited B-hCLDN18.2 MC38 tumor growth in C57BL/6 mice. B-hCLDN18.2 MC38 were subcutaneously implanted into C57BL/6 mice (n=7). Mice were grouped when tumor volume reached approximately 100 mm3, at which time they were treated with anti-human CLDN18.2 antibody with doses and schedules indicated in panel A. (B) Body weight changes during treatment. As shown in panel A, anti-human CLDN18.2 antibody was efficacious in controlling tumor growth in C57BL/6 mice, demonstrating that the B-hCLDN18.2 MC38 provide a powerful preclinical model for in vivo evaluation of anti-human CLDN18.2 antibodies. Values are expressed as mean ± SEM.
In vivo efficacy of anti human CLDN18.2-ADC drugs
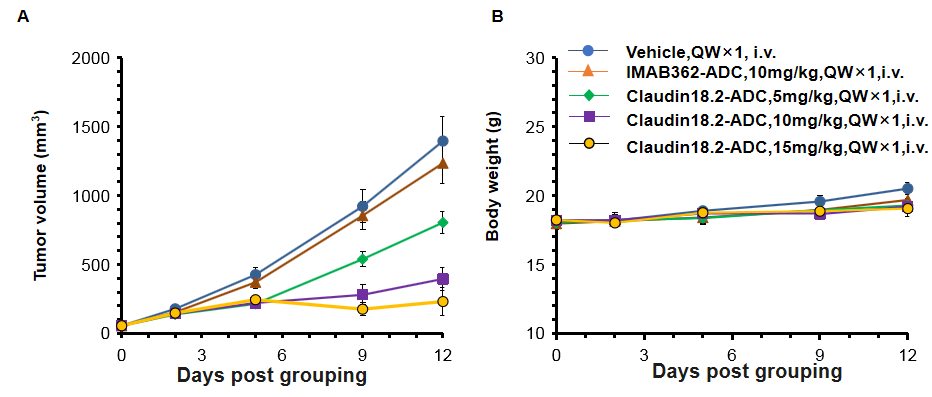
Antitumor activity of anti human CLDN18.2-ADC drugs in C57BL/6 mice. (A) Anti human CLDN18.2-ADC drugs inhibited B-hCLDN18.2 MC38 tumor growth in C57BL/6 mice. B-hCLDN18.2 MC38 were subcutaneously implanted into C57BL/6 mice(n=6). Mice were grouped when tumor volume reached approximately 100 mm3, at which time they were treated with anti human CLDN18.2-ADC drugs with doses and schedules indicated in panel A. (B) Body weight changes during treatment. As shown in panel A, anti human CLDN18.2-ADC drugs was efficacious in controlling tumor growth in C57BL/6 mice, demonstrating that the B-hCLDN18.2 MC38 provide a powerful preclinical model for in vivo evaluation of anti human CLDN18.2-ADC drugs. Values are expressed as mean ± SEM.