Biocytogen (HKEX: 02315) will attend the upcoming 42nd J.P. Morgan (JPM) Healthcare Conference and participate in several satellite conferences, which will be held in San Francisco from January 7-12, 2024.

Dr. Chaoshe Guo, Vice President and Global BD Head of Biocytogen, will be onsite with members of the Business Development & Licensing (BDL) team to discuss and explore partnerships for Biocytogen’s fully human antibody platforms and sequence library. Meetings will take place at the below events:
- Jan. 7: 7th Annual BFC Global Healthcare BD & Investment Conference
- Jan. 8-11: 42nd Annual J.P. Morgan Healthcare Conference
- Jan. 8-10: Biotech Showcase
- Jan. 8-12: BIO Partnering @ JPM Week
- Jan. 9-10: Fierce JPM Week
Our Discovery Platforms
Biocytogen officially launched the RenMice® series in 2023, which are powerful platforms used for fully human antibody/TCR discovery.
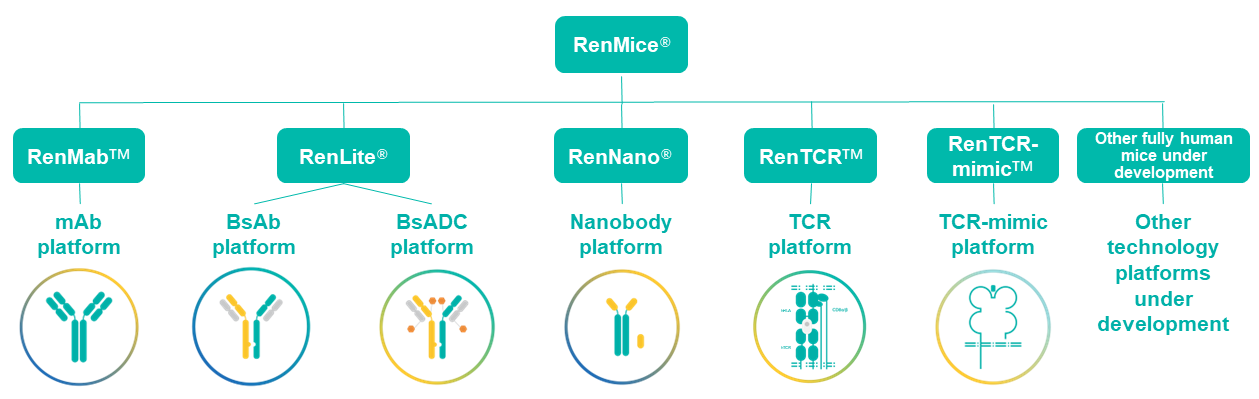
Overview of Biocytogen’s RenMice Antibody/TCR Platforms. These powerful platforms have been used to generate a fully human antibody library available for licensing and partnerships.
RenMice include:
- RenMabTM platform, designed for discovery of monoclonal antibodies (mAbs) with high specificity, affinity and diversity;
- RenLite® platform, designed for common light chain bispecific antibody (bsAb) discovery with high pairing success rate and good druggability, and for high-throughput bispecific ADC (bsADC) discovery for 200+ TAA targets with good tumor specificity, anti-tumor activity, and CMC efficiency;
- RenNano® platform for the discovery of fully human heavy-chain-only antibodies (HCAbs) or nanobodies to penetrate the blood-brain-barrier or infiltrate tumors, as well as facilitating the assembly of bispecific/multi-specific antibodies, CAR-T therapies, and other antibody-based modalities;
- RenTCRTM platform to discover novel fully human TCRs;
- RenTCR-mimicTM platform to enable discovery of TCR-mimic (TCRm) antibodies with high affinity and specificity to intracellular targets.
Biocytogen has leveraged RenMice platforms to perform 900+ target-specific antibody discovery projects, establishing a library of 400,000+ fully human antibody sequences with characterization data available for immediate evaluation and partnerships.
Assets Available for Licensing/Co-Development
Preclinical assets include:
- 10+ fully human bsAbs, including YH006 (CTLA-4×OX40 bsAb), 4-1BBxCD40 bsAb, and CD70xEGFR bsAb.
- 25+ fully human bsADCs, including DM001 (EGFRxTROP2 bsADC), DM002 (HER3xMUC1 bsADC), DM005 (EGFRxMET bsADC), BSA01 (EGFRxMUC1 bsADC), and BCG019 (EGFRxHER3 bsADC). Preclinical in vivo and in vitro anti-tumor activity data for bsADCs with proprietary linker/payload drug (BLD1102) were disclosed at SITC2023.
- 50+ targets with ongoing fully human HCAb/nanobody programs, including TFR1, 4-1BB, CD3, and BCMA. Payload brain delivery capacity of BBB-crossing TFR1 HCAbs was presented at the CNS drug delivery summit 2023;
- 10+ fully human TCRm antibodies against intracellular targets. Preclinical specificity and efficacy data for KRAS engager and NY-ESO-1 cell therapy was presented at SITC2023; preclinical in vivo and in vitro data for WT1xCD3x4-1BB tri-specific T cell engager co-developed with CtM Bio has been accepted for AACR2024 presentation.
- 60+ GPCRs with ongoing fully human mAb/bsAb programs, including CCR8, LGR5, CCR2, CXCR4, SSTR2, and GPRC5D;
- 900+ fully human antibody discovery projects targeting TNFR2, B7-H3, Siglec-15, BTN3A1, FAP, IL-2RA, LILRB4, M-CSF, MUC16, NKG2D, ROR1 and other therapeutic targets for oncology, inflammatory and autoimmune diseases, infectious diseases, cardiovascular and metabolic diseases, and neurological disease applications. Some of these assets have been presented at AACR2023.
Clinical assets include:
- YH003, a CD40 mAb in phase II MRCTs; complete phase I clinical data was presented at ESMO2023;
- YH001, a CTLA-4 mAb in an ongoing phase I clinical trial for the treatment of sarcoma, developed in partnership with TRACON;
- YH008, a PD-1×CD40 bsAb, which received both FDA and China NMPA IND clearance, is being developed in partnership with Chipscreen NewWay;
- YH002, an OX40 mAb in phase I clinical trials in Australia and China; YH002 and multiple other clinical-stage antibodies are in clinical trials in partnership with Syncromune for the development of intratumoral immunotherapy;
- YH004, a 4-1BB mAb in phase I clinical trials in Australia and China.
Worldwide Partnerships
Biocytogen’s therapeutic discovery platforms and assets have received recognition from biopharmaceutical and biotech companies around the world, including Merck KGaA, Janssen, Chipscreen NewWay, Neurocrine Biosciences, Ona Therapeutics, Myricx Bio, Pheon Therapeutics, ADC therapeutics, TRACON, Xencor, BeiGene, and Remegen. With a vast library of off-the-shelf fully human antibodies and a growing number of fully human drug discovery platforms, Biocytogen is committed to accelerating development of novel antibody-based therapeutics to improve human health worldwide.
Contact BD-Licensing@biocytogen.com to set up a meeting and discuss licensing and co-development opportunities!











