
Biocytogen’s antibody BD and licensing team is pleased to attend and host 1-on-1 meetings at BIO-Europe Spring, taking place March 18-20, 2024 in Barcelona, Spain.

Biocytogen’s antibody therapeutics R&D division, RenBiologics, offers antibody sequence licensing/co-development, and RenMice® fully human antibody/TCR discovery platform licensing opportunities.
RenBiologics: Our Fully Human Library. Your Pipeline.
Our team of antibody discovery experts has established a library of over 400,000 fully human antibody sequences against hundreds of targets. This antibody library is available for partners to develop different therapeutic modalities to treat cancer, autoimmune diseases, metabolic diseases, neurological diseases, and other diseases.
Antibodies and antibody-based assets available for partnership:
- 40+ PCC-stage antibody assets: B7-H3, Siglec-15, TNFR2, MUC16, NKG2D, ROR1, TIGIT, IL-2RA, AMHR2, CD73, CD40 (antagonist), and more;
- 70+ TAA antibodies for plug-and-play: MUC1, 5T4 (TPBG), PTK7, FOLR1, CEACAM6, PSMA, B7-H3, CDH3, DLL3, SEZ6, HER3, HLA-G, LIV-1, ROR1, MUC16, and more;
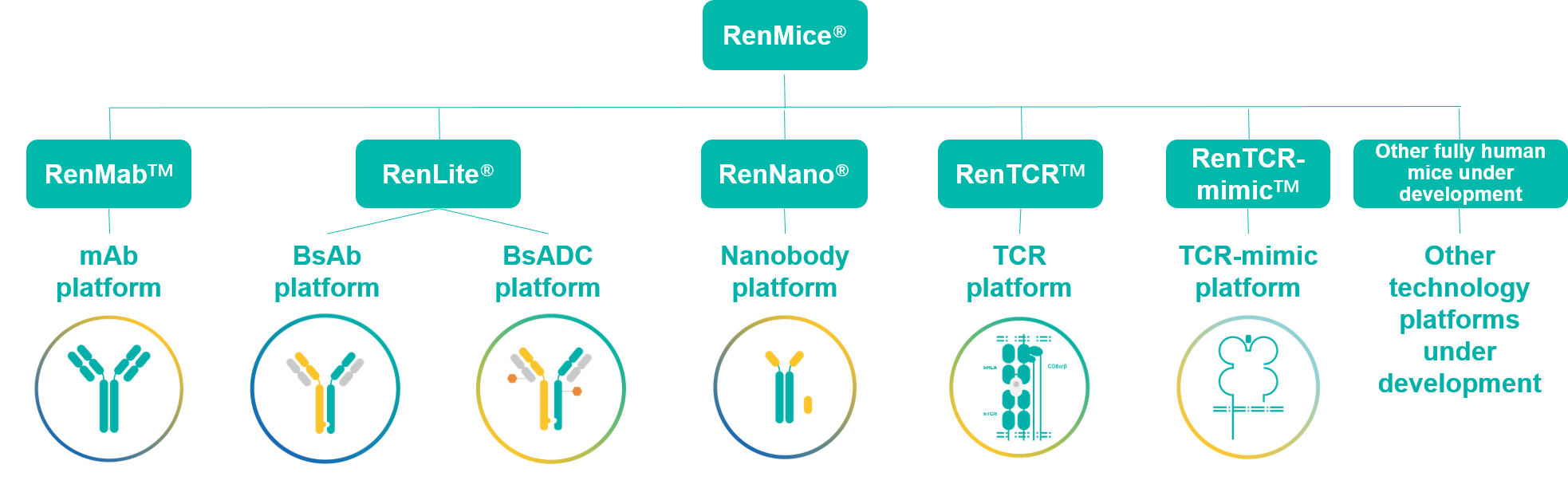
- 10+ fully human bsAb: RenLite® derived 4-1BB x CD40 bsAb for oncology, OX40 x OX40 (biparatopic) bsAb for autoimmunity, and more;
- 20+ bsADC: RenLite derived PTK7 x TROP2 bsADC, HER3 x MET bsADC, PTK7 x EGFR bsADC, PTK7 x B7-H3 bsADC, and more;
- 10+ TCR-mimic antibodies: RenTCR-mimicTM derived antibodies against WT1、KRAS、P53、AFP、GP100、NY-ESO-1 and other intracellular targets;
- 50+ nanobodies and Nano 100 Project: RenNano® derived HCAbs/nanobodies against TFR1 (blood-brain-barrier crossing), 4-1BB, OX40, BCMA, and more;
- 60+ fully human GPCR mAb/bsAb programs: CCR8, GPRC5D, LGR5, CCR2, and more.
5 clinical-stage assets available for partnership:
- YH008, a PD-1×CD40 bsAb, which received both FDA and China NMPA IND clearance, is being developed in partnership with Chipscreen NewWay;
- YH003, a CD40 agonistic mAb in phase II MRCTs for unresectable/metastatic pancreatic ductal adenocarcinoma (PDAC) and phase II in China for unresectable/metastatic mucosal melanoma;
- YH001, a CTLA-4 mAb in an ongoing phase I clinical trial for the treatment of sarcoma, developed in partnership with TRACON;
- YH002, an OX40 mAb in phase I clinical trials in Australia and China; YH002 and multiple other clinical-stage antibodies are in clinical trials in partnership with Syncromune for the development of intratumoral immunotherapy;
- YH004, a 4-1BB mAb in phase I clinical trials in Australia and China.
Biocytogen’s therapeutic antibodies and RenMice discovery platforms have received recognition from biopharmaceutical and biotech companies around the world. As of Dec. 31, 2023, 103 therapeutic antibody and multiple clinical asset co-development/out-licensing/transfer agreements and 47 target-nominated RenMice licensing projects have been established around the globe, including several partnerships with multinational pharmaceutical companies (MNCs). Biocytogen will continue to actively seek partnerships to accelerate the development of novel therapies for the benefits of human health worldwide.
Contact BD-Licensing@biocytogen.com to set up 1-on-1 meetings and discuss licensing and co-development opportunities!











