BCG003 is a fully human monoclonal antibody targeting TNFR2, which was co-developed by Biocytogen and Dragon Boat Pharmaceutical. The preclinical results showed that BCG003 is an agonistic TNFR2 antibody without blocking the TNFα-TNFR2 interactions. BCG003 has a favorable anti-tumor activity in many syngeneic mouse tumor models. In addition, BCG003 can enhance the anti-tumor activity of immune checkpoint blockades in TNFR2 humanized mice with good safety profile.
Preclinical results:
1. Treatment with BCG003 reduced tumor volumes in multiple tumor models, including colon cancer, glioma, and melanoma models.
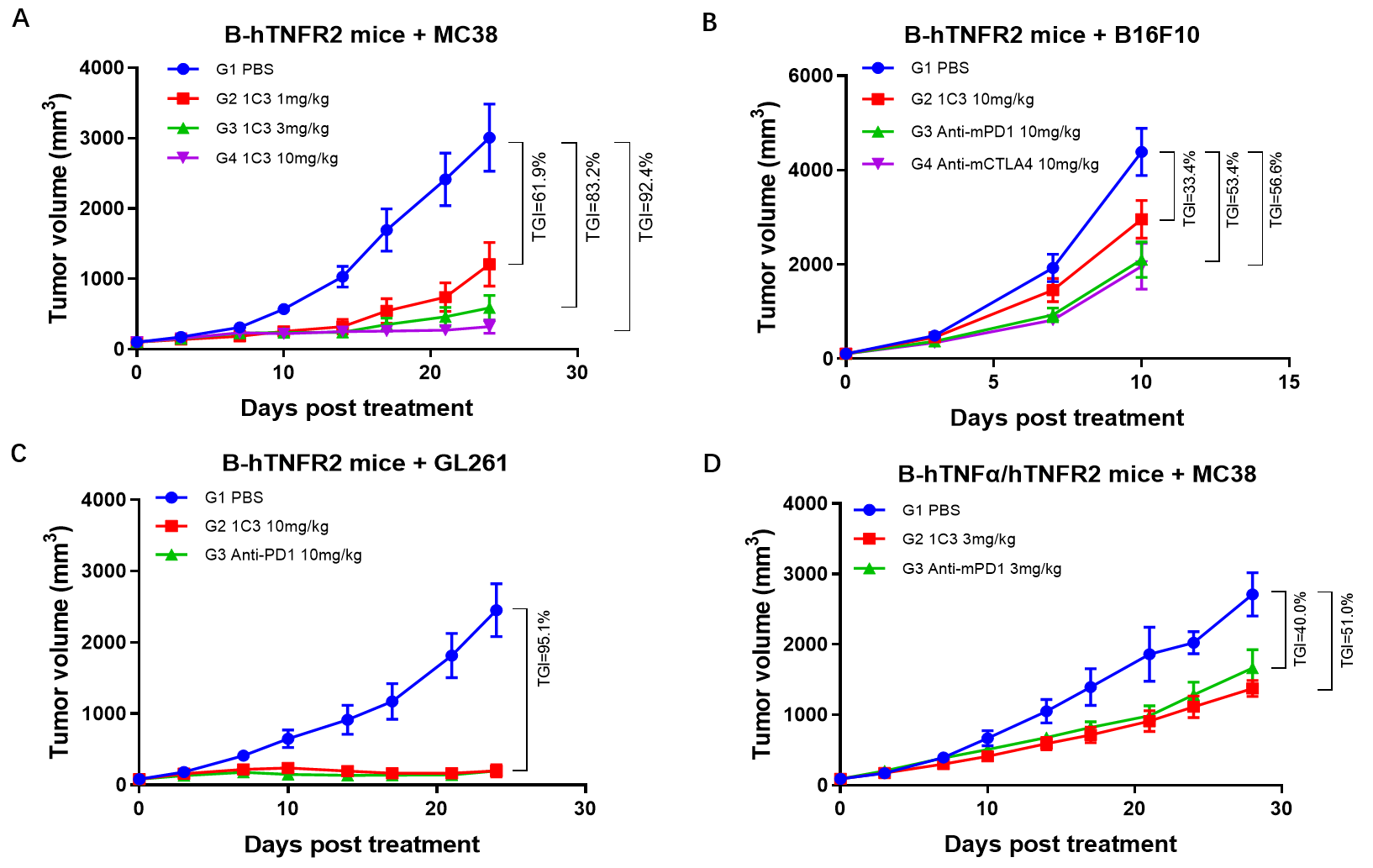
Figure 1. BCG003 showed anti-tumor activity in multiple tumor models.
2. Treatment of BCG003 enhanced the efficacy of PD-1/PD-L1 blockade in MC38 model
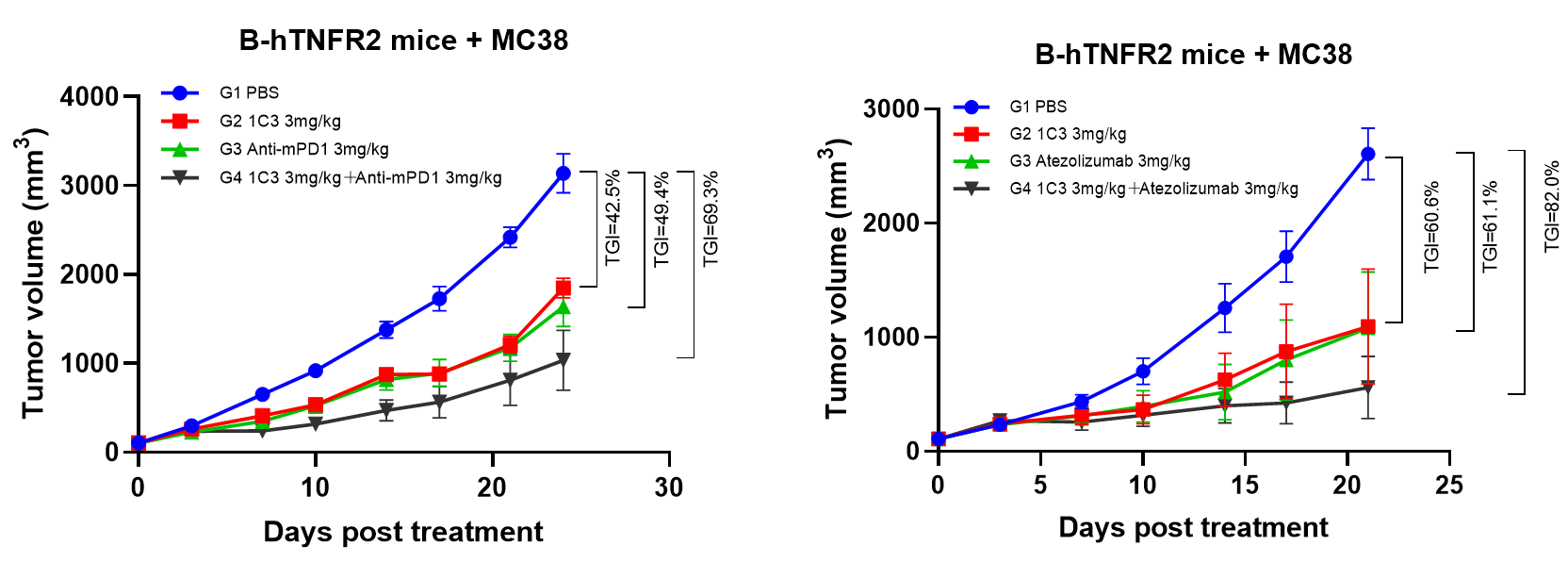
Figure 2. BCG003 has synergistic effect when combined with immune checkpoint blockades.
3. BCG003 is an IgG1 antibody with ADCC activity. In addition, BCG003 can enhance the CD8+ T cell proliferation. In vitro experiment showed that BCG003 treatment significantly increased the Teff/Treg ratio in tumor-microenvironment.
4. BCG003 was well tolerated in B-hTNFR2 mice. No toxicity showed at the dose of 100 mg/kg.
Target TNFR2
Tumor necrosis factor receptor-2 (TNFR2) is selectively highly expressed on immune cells and some tumor cells, which mediates the signaling of immune stimulation and survival, which result in cell activation, migration and proliferation. Research indicates that TNFR2 plays an important role in tumor immune escape and tumor growth.
Previous research has showned that TNFR2 antibody is as effective as PD-1 antibody. In addition, TNFR2 antibody also has anti-tumor effect in PD-1 resistant tumor models and the effect is even stronger when two drugs combined. Therefore, TNFR2 has become a next generation cancer therapy.
So far, the research and development of drugs targeting TNFR2 is still at an early stage worldwide. There is no such product on the market yet. Most pipeline assets are at preclinical stage and the fastest at clinical phase 1/2a.
Poster Download
AACR2023: 1C3, A Novel Non-Blocking Anti-Human TNFR2 Antibody, Promotes Effector T Cell Responses and Demonstrates Potent Anti-Tumor Activity
AACR2022: 1C3, a Novel non-blocking anti-human TNFR2 antibody generated from RenMice, exhibits promising anti-tumor activity and safety in syngeneic tumor models in humanized TNFR2 mice













