CMC Development Platform
After developability assessment of PCC(Preclinical candidate compounds),we move on high-efficiency CMC development, including cell line development, small-scale process development, analytical methods development and formulation development . We have extensive experience in process development and transfer with multiple successful projects.
Developability assessment
We undertake the developability assessment work for the “project Integrum”. We have conducted developability assessment studies on 1000+ patented antibody molecules in house, including: sequence analysis, antibody hydrophobicity, PEG solubility, isoelectric point, antibody cross-interaction, colloidal stability, antibody surface charge, low pH stability, strong oxidation , deamidation , accelerated stability , freeze-thaw stability etc.. We also established internal standards for antibody developability assessment at Biocytogen based on these data and industry experience.

Cell Line development
We possess a CHO cell antibody expression system with GS knockout, capable of expressing various forms of antibodies with an expression level between 6-12g/L. Equipped with advanced facilities and equipment, we have established a complete stable cell line construction process based on industry-leading experience.
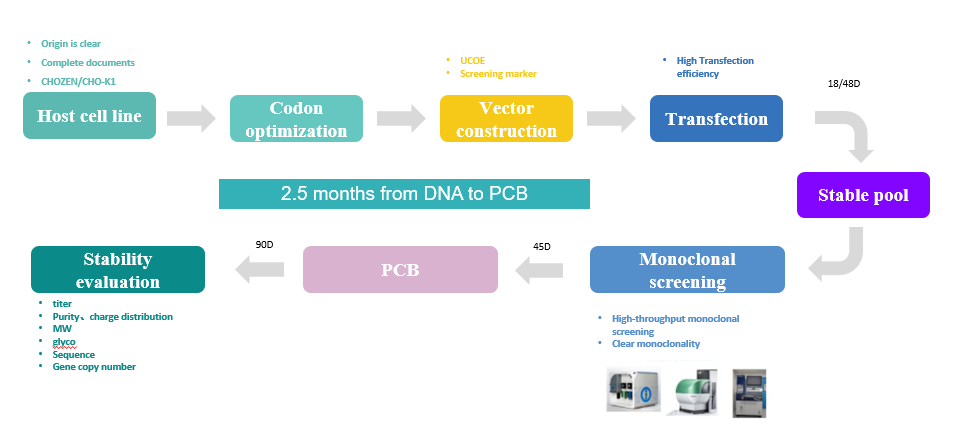
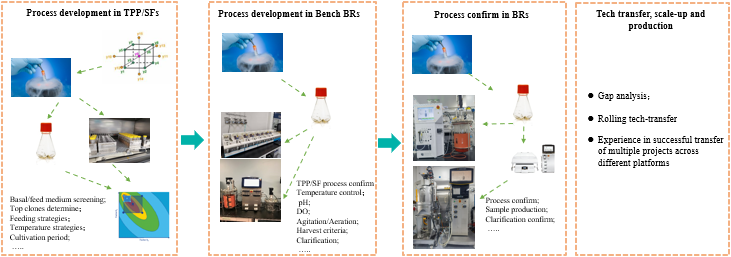
Cell culture process development
1)50mLTPP-Ambr 250mL-Applikon 3L/Sartorius 2L-Applikon 15L/Sartorius RM-STR50
2)Process control equipments : Roche CEDEX ,Siemens BGA, Gonotech Osm, Countstar etc
3)Mature platform technology and Core employees with over 10 years of experience
4)Stable cell performance
5)High expression level
6)IPT:cell VCD、VIA、Diameter、Osm、Glucose、lac、titer、pCO2、PO2,etc
7)Quality
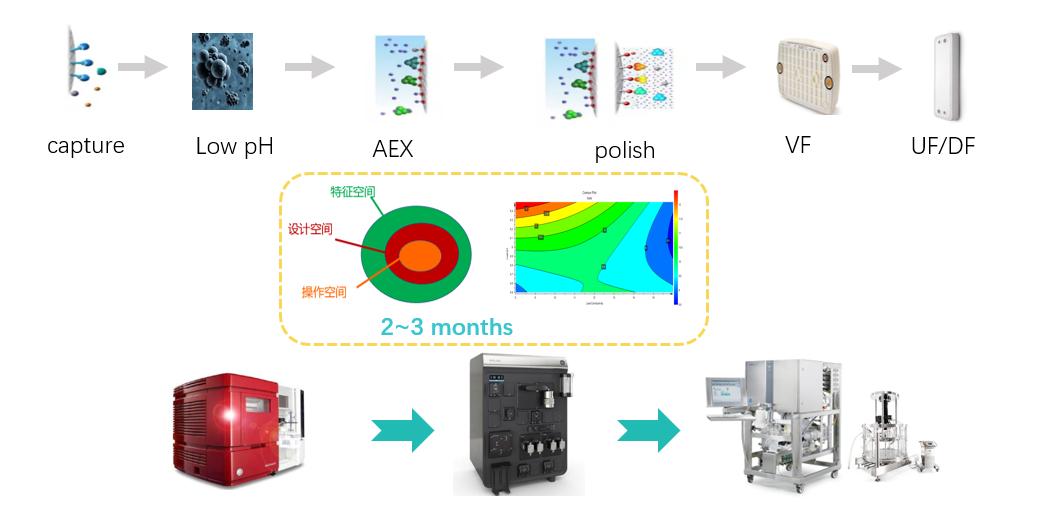
Downstream process development
1) Rich resins and consumables inventory, 10+AKTA Avant/Purifier/Pilot
2) Platform process to accelerates project development
3) Efficient experimental design improves development efficiency
4) Perfect document system and rich experience in technology transfer
ADC conjugation
1) Self-owned property hydrophilicity linker-payload
2) 13+ ADC process development experience(DAR4/DAR8)
3) various linker-payloads experience to mg->100mg->10g scale up
4) Preparation of early pharmacological and toxicological samples
5) fast process development

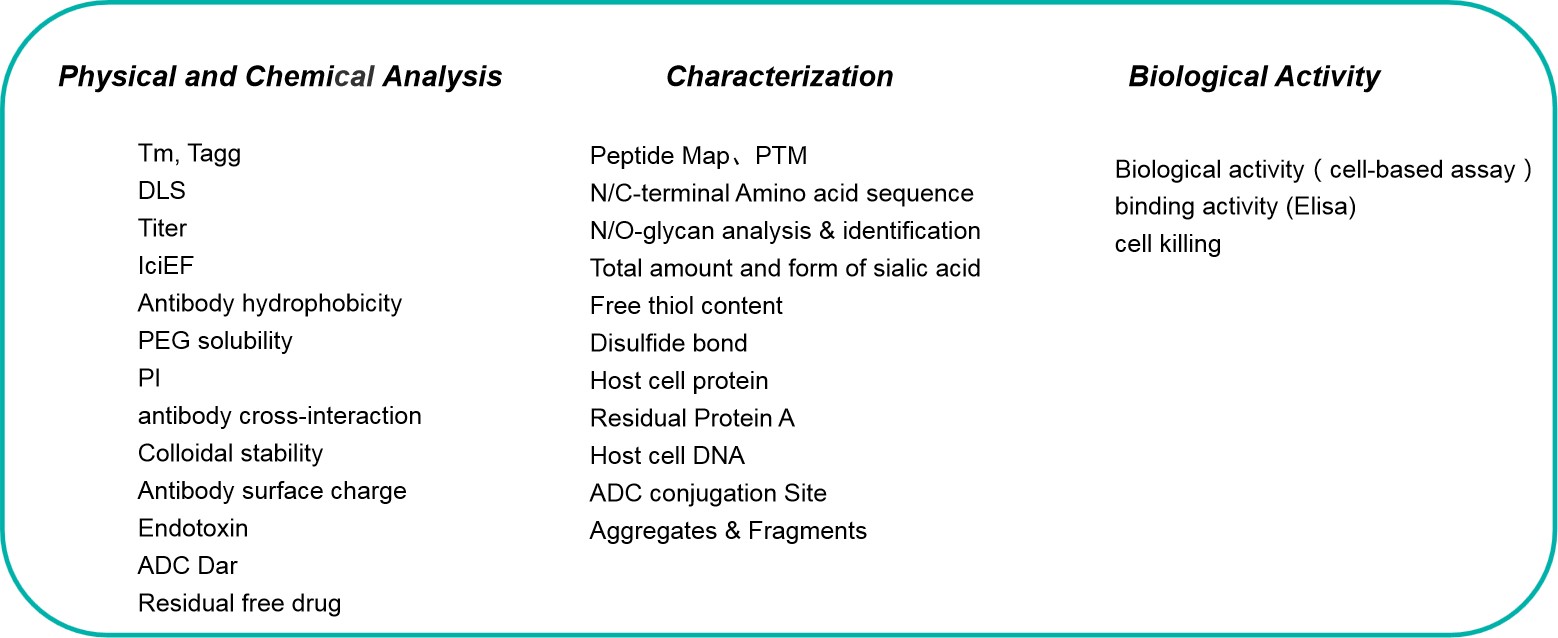
Analysis and Formulation
1) Analysis methods development: evaluate platform methods based on developability assessment.
2) Biological activity development: Binding Elisa; cell-based/cell killing established with cell resource library at Biocytogen.
3) High concentration liquid formulation development.
4) Lyophilized formulation process development.